Battery chemistry advance promises to deliver 'smaller, lighter, and cheaper' Li-ion cells
Cathode hydrogenation identified as the primary cause of lithium battery degradation.
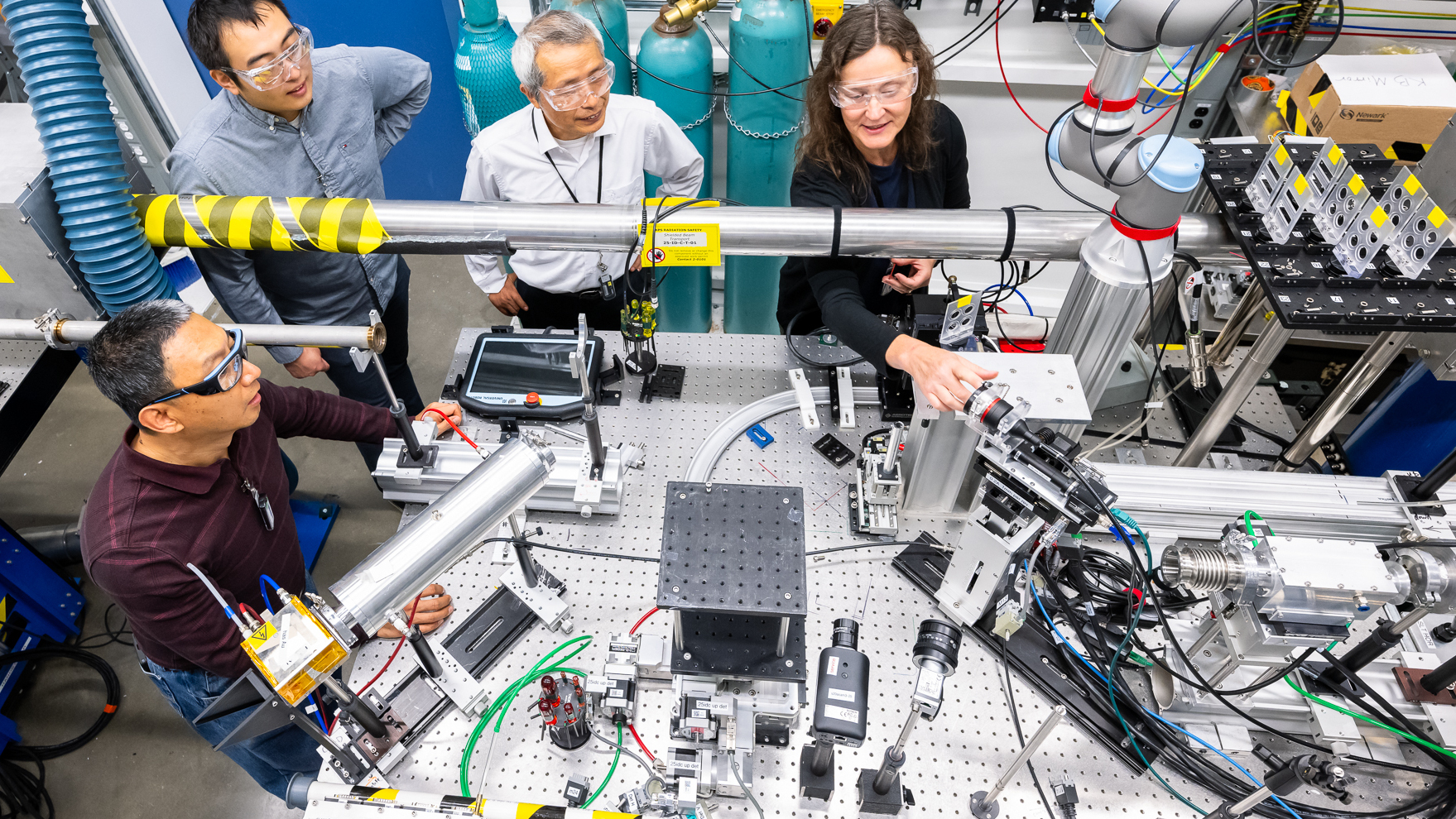
An advance in battery chemistry promises to deliver a "smaller, lighter, and cheaper battery without sacrificing end-of-life battery performance." On September 13, researchers from around the United States released a paper in collaboration with the Department of Energy's Argonne National Laboratory titled "Solvent-mediated oxide hydrogenation in layered cathodes", with additional comments and a summary in an official press release on the Argonne National Laboratory website. In short, the researchers used the cutting-edge X-ray technology available at Argonne National Laboratory and its Advanced Photon Source, which pairs X-ray studies and electrochemistry to allow researchers to examine their batteries at a molecular level.
But why go through all this trouble? You may or may not know this, but the rechargeable batteries available in your phones, electric vehicles and such are most likely lithium-ion batteries. Lithium-ion batteries degrade over time, getting steadily worse and worse at holding charge until they simply no longer function or can't hold a charge for a meaningful amount of time. Just because we've known how to make lithium-ion batteries for so long doesn't mean we've perfected them — advancements into "perfection" require deeper study than just being able to make a functioning item.
According to Argonne Senior Chemist Zonghai Chen, "Self-discharge is a phenomenon experienced by all rechargeable electrochemical devices. The process slowly consumes precious functional battery materials and deposits undesired side products on the surface of the battery components. This leads to continuous degradation of battery performance."
In this case, using high-end equipment allowed researchers to bridge a key missing link in existing research by getting an otherwise impossible molecular view of a lithium-ion battery in action. This allowed researchers to identify a specific process: cathode hydrogenation, which is the process of "dynamically transferring the protons and electrons from the electrolyte solvent into highly charged layered oxides in the cathode". The chemical reaction here leads to battery degradation, and while lithium-ion batteries have been known to have degradation issues for a long time, this greatly narrows down the cause.
So, what does it mean in the long term? As Zonghai Chen says, "By mitigating self-discharge, we can design a smaller, lighter, and cheaper battery without sacrificing end-of-life battery performance." This should be a pretty big step toward a future where rechargeable batteries are not awful. Here's hoping!
Get Tom's Hardware's best news and in-depth reviews, straight to your inbox.

Christopher Harper has been a successful freelance tech writer specializing in PC hardware and gaming since 2015, and ghostwrote for various B2B clients in High School before that. Outside of work, Christopher is best known to friends and rivals as an active competitive player in various eSports (particularly fighting games and arena shooters) and a purveyor of music ranging from Jimi Hendrix to Killer Mike to the Sonic Adventure 2 soundtrack.
-
thisisaname "Solvent-mediated oxide hydrogenation in layered cathodes", sounds like water is getting in and being split to H2 and O. The Oxygen is causing the Oxide and the H2 the Hydrogenation. Easy to say, may be quite hard to fix.Reply -
blazorthon Reply
Lithium ion battery cathodes are made of oxides and most solvents contain hydrogen. Most organic solvents contain oxygen. Water is almost certainly not related to anything going on inside a lithium ion battery in any significant way.thisisaname said:"Solvent-mediated oxide hydrogenation in layered cathodes", sounds like water is getting in and being split to H2 and O. The Oxygen is causing the Oxide and the H2 the Hydrogenation. Easy to say, may be quite hard to fix. -
palladin9479 We've kinda suspected this as the primary issue with LiION technology for awhile, good to see it demonstrated conclusively. This is why I won't purchase any battery powered passenger vehicles, battery degradation is a huge issue in the long term operation of said vehicles. Reducing this effect by an order of magnitude would go a very long way to making it more then a niche product.Reply -
Eximo My old Focus EV was starting to show signs of its 8+ years. But only about 15% less capacity it seemed to me. Considering it only started out with 76 miles of range, that was problematic for my out of town trips. No fast charging on that model either and it was always topped up to 100%, which was less than ideal.Reply
But if I take the more typical EV capacity of around 250 miles range and consider a low estimate of 500 charge cycles before significant issues. That is still 125,000 miles range. I certainly do under 10k miles per year average since I have a relatively short commute. So 8 years does look to be about right again before I see significant degradation. But even at 15-20% less capacity, that would still be a perfectly viable car for my use.
Perfectly viable for anyone that doesn't do daily long distance commuting at highway speeds.
By then I expect some base range increase and or other features that will be worth looking at in 8-10 years. -
Notton My Prius hybrid is >12 years old. Battery degradation is about 15~20%, but it's basically unnoticeable. The range on a full tank is only around <8% less compared to 8 years ago.Reply
The e-scooter I have is too new to see any range loss.
I notice battery degradation more on phones, with a larger annoyance against counterfeit replacement batteries for them. -
Amdlova My old galaxy s7 still working today with original batery. first years of use of it make slow charger... today it is with my mom... she charge it with a nokia charge without quick charge. My s22 with quick charge the batery died within two years of use.Reply
Eletric cars it's not for me. I drive 650 Miles per year. Never will get the money of a new shine toy car. -
67Matt99 Reply
As will the US as 'They' continue to piece meal us out to China and who ever wins the auction sale.Pei-chen said:So the lab is 75% Chinese?….
It's been going on for quite some time now with our manufacturing, Big Pharma, Auto Industry etc.(Ivory Towers maybe in the US)but manufacturing is sent overseas like most of it has.
If China is the one the US is to fear, it's because We made it so. -
nameless0ne I am excited for the future. Vehicles with 10 year battery warranty and 15 year expected lifespan are starting to appear. If even this can be improved then it might persuade more people to switch.Reply
But a lot of EVs from the last decade will go out of warranty and will appear on second hand market. If those start to develop battery problems, then on a global scale - it might negatively impact EV viability perception.
And small devices from smartphones to earbuds would definitely benefit from better battery lifespan. Those tend to degrade faster because there is less leeway to optimally manage the battery. -
67Matt99 "If even this can be improved then it might persuade more people to switch."Reply
As long as their are doubts about the real impact of the EV battery mining, production and recycling having less impact on the planet there will those who will rather 'fight than switch'.
The National Grid System has not kept pace with present day demand. It's old and outdated.
That is a fact and the info to back it is readily available.