Cooler Master Demos 3D Vapor Chamber CPU Cooler Tech
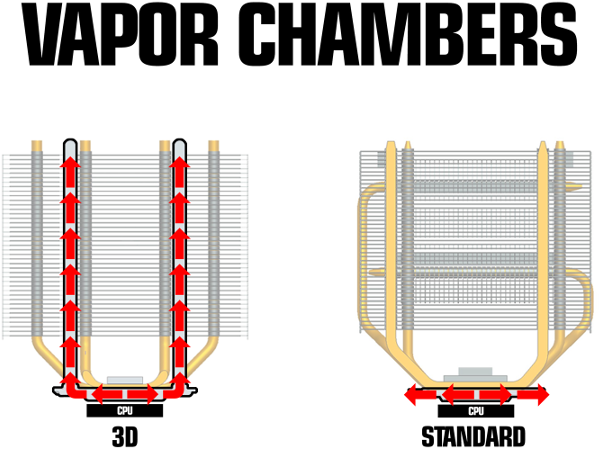
Cooler Master showed us its next generation CPU cooling technology at CES 2015, which it calls "3D Vapor Chamber." Simply put, it's an evolution of the company's vapor chamber technology. Vapor chambers for CPU coolers are sealed, flat-ish metal pockets filled with fluid. As the CPU heats the liquid in the vapor chamber, the liquid ensures that it is dissipated evenly to the copper heat pipes, thus eliminating hot spots and ensuring better cooling.
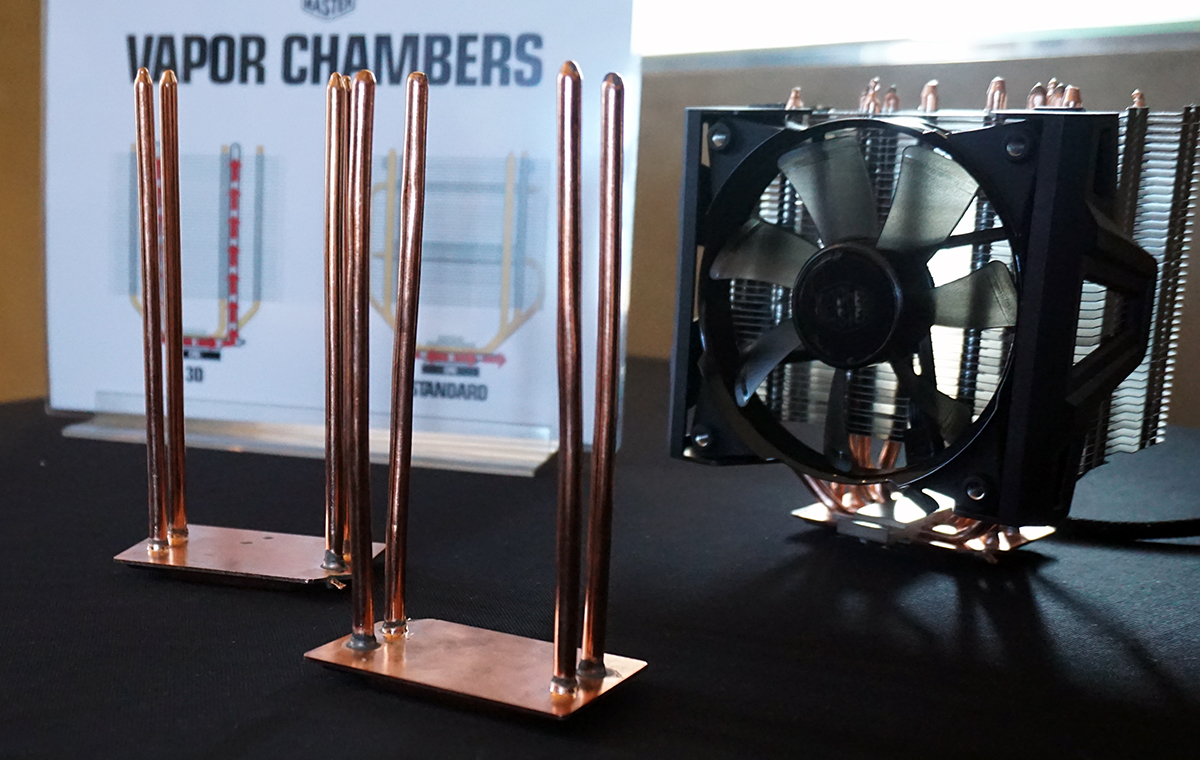
Cooler Master has taken the concept to the next level by effectively making the heat pipes part of the actual vapor chamber. Instead of laying on top of the vapor chamber, the heat pipes are connected to it. The heatsink and heat pipes create a closed vapor chamber system. Thus, the warmed liquid is run up through the fin assembly of the cooler.
The idea is straightforward, and it's reasonable to expect this 3D Vapor Chamber technology to maximize the cooling efficiency of an air cooler.
Currently, though, all Cooler Master has is some basic mockups of how a 3D Vapor Chamber would look with the pipes connected to the heat sink vapor chamber, but we expect the company to have working prototypes, if not market-ready products, by Computex later this year.
When we asked about the potential issue of the pipes' rigidity due to the possible lack of internal reinforcement, Cooler Master told us that we should expect the same rigidity as regular copper heat pipes. However, a company representative also noted that Cooler Master has yet to finalize a manufacturing process, so we should perhaps assign a small asterisk next to that claim.
Still, Cooler Master is an OEM, so the company can leverage its own internal process technology to quickly churn through prototypes without relying on a third party.
Follow Seth Colaner @SethColaner. Follow us @tomshardware, on Facebook and on Google+.
Get Tom's Hardware's best news and in-depth reviews, straight to your inbox.
Seth Colaner previously served as News Director at Tom's Hardware. He covered technology news, focusing on keyboards, virtual reality, and wearables.
-
jossrik I thought the problem with vapor chambers was the direction they were facing. In the picture it looks all good, but in reality that cooler would be sideways, so wouldn't the vapor condense on the side (bottom) of the cooler? Same problem for video cards, I understand the tech, with the way video cards sit in a case, the heat source is the top of the cooler, and for a liquid vapor chamber you'd need the bottom for the liquid, there aren't any liquids that are lighter than their vapor counterparts... Am I missing something?Reply -
dwatterworth ReplyHow is this any different than a direct-contact heatpipe?
It's different from a direct contact heatpipe in the way that the whole CPU lid would be covered evenly, instead of problems faced with heatpipe orientation, missed CPU hotspots etc. Even DC heatpipes that are mashed together will have different heat transfer rates where the heatpipes merge and form thicker copper sections. -
Simon Ayres The orientation will have a minimal effect on the vapor chambers, the second law of thermodynamics and the zeroth law of thermodynamics stat that everything moves toward thermal equilibrium.Reply
The reason heat rises is due to the fact that anything with more "heat" or energy is less dense then the same type of matter with less energy making the earths gravitational pull on it lower.
But no matter what the heat will still spread even if the heat source is on top because energy by nature radiates away from its source. -
Ron Olbrey Actually, for heat pipes under a foot or two in length, orientation does not matter. The fluid is held in a metal sponge lining on the entire inside surface of the pipe, so all parts of the pipe are in contact with liquid. The heat source boils the liquid off at the heat contact point, this vapor travels through the pipe and condenses everywhere else, releasing heat. Fluid is wicked back to the heat source through capillary action of the sponge.Reply
Heat is transferred not by flowing fluid, but by the vapor pressure increase caused by the boiling of the liquid at the heat point. The heat travels to every point in the pipe at the speed of the pressure wave, essentially the speed of sound.
-
yhikum This is somewhat confusing.Reply
The so called "vapor" would carry a unit of thermal energy to the point where "vapor" can expand into, most likely, to the opposite end of metal tube where "vapor" is held. This is in assumption that "vapor" can travel there, that vaporized liquid is moving inside sealed enclosure.
Now, what happens when you heat up liquid inside sealed enclosure? A liquid would start expanding into a gas, creating internal pressure several orders of magnitude of starting pressure (if it can expand). The case can be easily demonstrated with steam engines or frozen water, where in both cases base liquid expands to create more pressure than at liquid state.
At some point of time thermal saturation can be reached to have all of the liquid converted into gas (again, if pressure is maintained boiling point of liquid will change dramatically, so higher temperatures are required for liquid -> gas conversion), at which point we have some nice steam pipe about to burst into small explosion due to all of the accumulated pressure.
In short, I find it hard to believe that a small sealed system would be used to convert liquid into vapor just to transfer heat over short distance due to all of the pressure involved. It would be believable to use liquid which can transfer heat much more efficiently than copper. -
dwatterworth Reply15015431 said:This is somewhat confusing.
The so called "vapor" would carry a unit of thermal energy to the point where "vapor" can expand into, most likely, to the opposite end of metal tube where "vapor" is held. This is in assumption that "vapor" can travel there, that vaporized liquid is moving inside sealed enclosure.
Now, what happens when you heat up liquid inside sealed enclosure? A liquid would start expanding into a gas, creating internal pressure several orders of magnitude of starting pressure (if it can expand). The case can be easily demonstrated with steam engines or frozen water, where in both cases base liquid expands to create more pressure than at liquid state.
At some point of time thermal saturation can be reached to have all of the liquid converted into gas (again, if pressure is maintained boiling point of liquid will change dramatically, so higher temperatures are required for liquid -> gas conversion), at which point we have some nice steam pipe about to burst into small explosion due to all of the accumulated pressure.
In short, I find it hard to believe that a small sealed system would be used to convert liquid into vapor just to transfer heat over short distance due to all of the pressure involved. It would be believable to use liquid which can transfer heat much more efficiently than copper.
Check this website which explains how heat pipes work. There is also an explanation of water heat pipes. I think your primary confusion is coming from your phasing for the manufacturing process and quantities used.
http://en.wikipedia.org/wiki/Heat_pipe -
Ron Olbrey Most copper heat pipes use water as a fluid. The key is that they only contain water, no air. As such, at room temperature, the pressure is very low. The pressure only gets to normal atmospheric pressure at 100C, or 212F.Reply
The important point is that any enclosed space with only water, and water vapor, no other gasses, the water will be at its boiling point all the way down to near freezing. So at room temperature, any added heat causes the water to boil, creating vapor (which the transition from, for instance, 25C liquid to 25C gas requires a great deal of energy and absorbs heat).
The 25C gas created increases the pressure in the entire pipe, evenly and instantly, which causes the boiling point to go up. This causes vapor over the entire unheated part of the pipe to condense to a liquid, thereby releasing heat.
The fascinating fact is that the heat absorbed by the liquid to gas transition at one end, is released by a gas to liquid transition at the other end by the gas already at the other end, the heat is transferred by the pressure increase, not by the transfer of a heated material. Also, the heat transfer does not require the media to increase in temperature, only to change phase, which occurs at a constant temperature. Hence the very low thermal resistance.