In Pictures: Tom's Hardware Recovers Gold And Silver From CPUs
Processors are responsible for operating on all of your precious data. But did you know that they also are made up, in part, of precious metals? We're going to show you how to recover the gold and silver hidden away inside your old CPUs.
Meet Our Mountain Of CPUs
A while back, we did a little do-it-yourself salvaging of gold from motherboards (Can You Mine Gold From Old Motherboards?). But that’s not the only part of a computer where the rustless, unalterable metal is used. It’s also in CPUs. Specifically, the element is used on the pins and on the mounting pads of the processor dies. Also, the small wires that connect the pads and the pins are made of gold.
Here's the hard part, though: recovering the precious metal through do-it-yourself techniques. In this piece, we're also going to recover another precious metal: silver.
Please note that the chemicals used in this demonstration are extremely dangerous, especially in the concentrations used. Therefore, we strongly discourage you from attempting to reproduce this experiment at home.
Nitric Acid
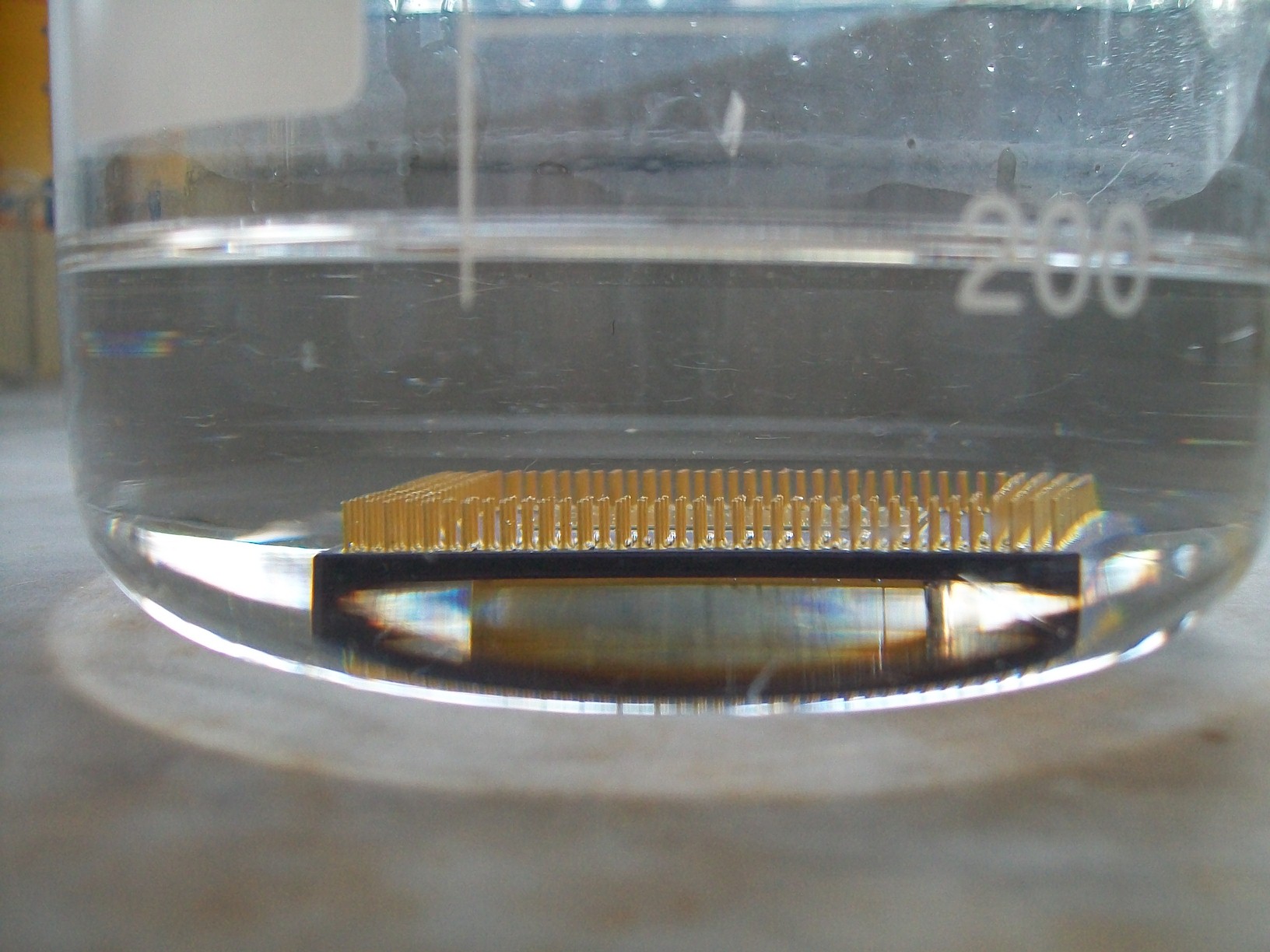
In our first step, the processors are dropped into a bath of concentrated nitric acid. They’re going to spend a while there, as we'll see. And the experience won't be particularly pleasant for them, either.
Splish Splash!
During this stage, the strong acid reacts with certain metals, particularly copper and silver. Nitric acid has no effect on gold, however.
Interestingly, our K5 doesn’t seem to react to the forced bath...yet.
Bath Time For Our CPUs
Let’s throw in a few more processors. On the left, our CPUs have just been tossed into a concentrated nitric acid solution. On the right, after a few minutes, the solution is already taking on a bluish color, characteristic of the presence of copper(II) nitrate, and thus of the Cu2+ ion. It's also important to note that the bath is now giving off a brown gas (nitrogen dioxide, or NO2).
Get Tom's Hardware's best news and in-depth reviews, straight to your inbox.
Cu + 4 HNO3 -> Cu(NO3)2 + 2 NO2 + 2 H2O
Attention: This Is Dangerous
The reaction can be more rapid (and violent) at times, but it’s always dangerous.
The Bath, A Few Weeks Later
After a few weeks, the solution is very blue, due to the strong presence of Cu2+ ions. The copper and silver (among other things) are dissolved, but not the gold.
CPUs: No Longer Functional
Now we remove the processors from the bath.Needless to say, they wouldn’t perform quite as well now...
Filtering
The next step is to filter. Silver is in the solution as silver nitrate (AgNO3), whereas the gold and a few other metals that weren't attacked by the nitric acid, plus the impurities, remain in the filter. We’ll set the filter aside for later and concentrate on the solution.
A Pinch Of Salt
We add a little sodium chloride (ordinary table salt) to the solution.
Silver Precipitates
It’s magic (well, almost)! The silver in the silver nitrate precipitates and forms silver chloride.
AgNO3(aq) + NaCl(Aq) -> AgCl(s) + NaNO3(aq)
A Little Zinc
Now we add a little hydrochloric acid. Then, we immerse a piece of zinc in the same solution. Attention: This is an exothermic reaction!
-
jprahman It's kind of sad to see these processors get destroyed, I mean some of those are classics that would be cool to have as a keepsake.Reply -
gmcizzle And here I thought high school chemistry was useless. Don't try this at home though...well unless you want to see how fast chlorine gas kills everything around you.Reply -
alhanelem i have an old pentium MMX on display (in my room on a shelf where all my unused computer hardware goes)Reply -
LuckyDucky7 Woah! Better keep those AMD CPU's- those are ancient relics of when AMD used to actively compete with Intel!Reply -
frostmachine Simple electrolysis can get it to even higher purity. 999 might be difficult but 916 n above should be easy. Good enough for a ring/pendant. Heck, with enough CPU u can even engraved "Intel Inside" :DReply